
文章:
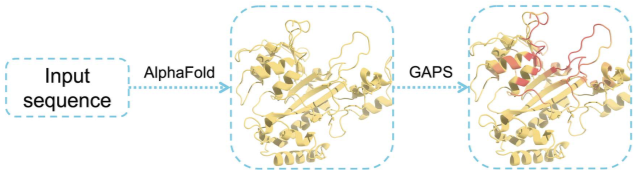
19. Zhu, C.; Zhang, C.; Shang, T.; Zhang, C.; Zhai, S.; Cao, L.; Xu, Z.; Su, Z.; Song, Y.; Su, A.; Li, C.; Duan, H.* GAPS: a geometric attention-based network for peptide binding site identification by the transfer learning approach. Brief. Bioinform., 2024, 25, bbae297.
18. Zhan, W.; Duan, H.; Li, C.* Recent Advances in Metal-Free Peptide Stapling Strategies. Chem Bio Eng., 2024, 1, 593–605. (Cover Art)
17. Miao, J.; Ghosh, A. P.; Ho, M. N.; Li, C.; Huang, X.; Pentelute, B. L.; Baleja, J. D.; Lin, Y.-S.* Assessing the performance of peptide force fields for modeling the solution structural ensembles of cyclic peptides. J. Phys. Chem. B2024, 128, 5281–5292.

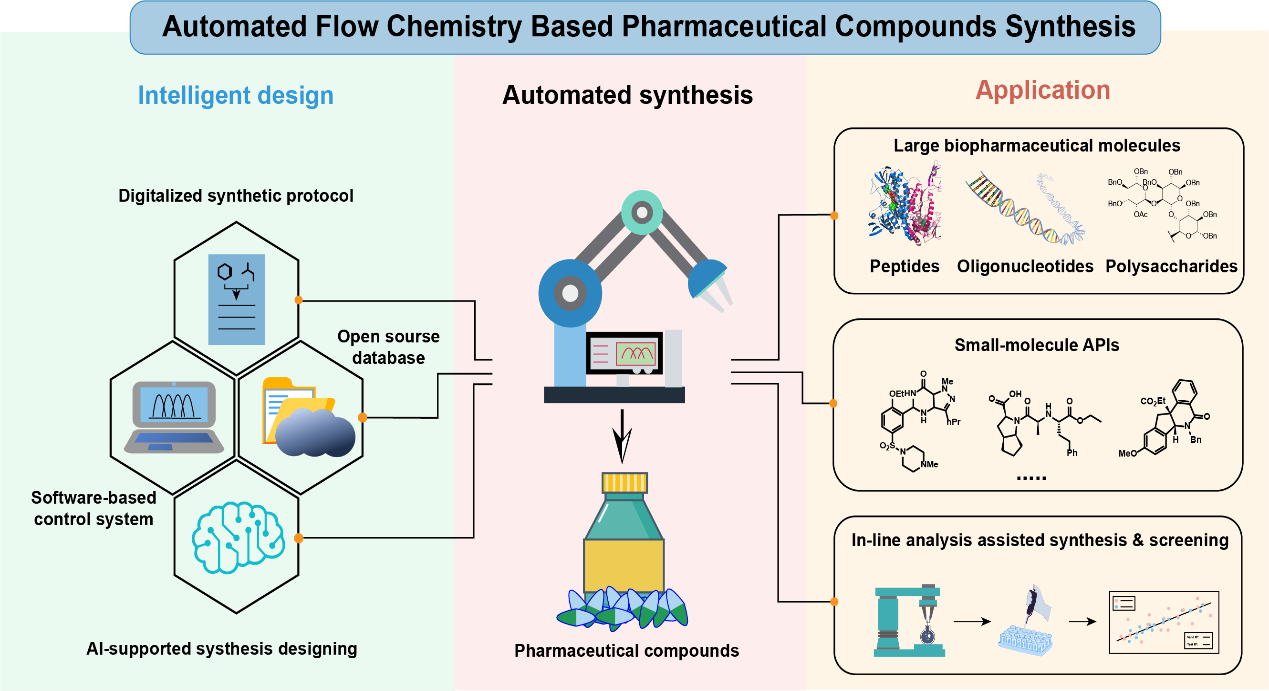
16. Wu, J.; Yang, X.; Pan, Y.; Zuo,T.; Ning, Z.; Li, C.* Zhang, Z.* Recent Developments of Automated Flow Chemistry in Pharmaceutical Compounds Synthesis. J. Flow. Chem., 2023, 13, 385-404.
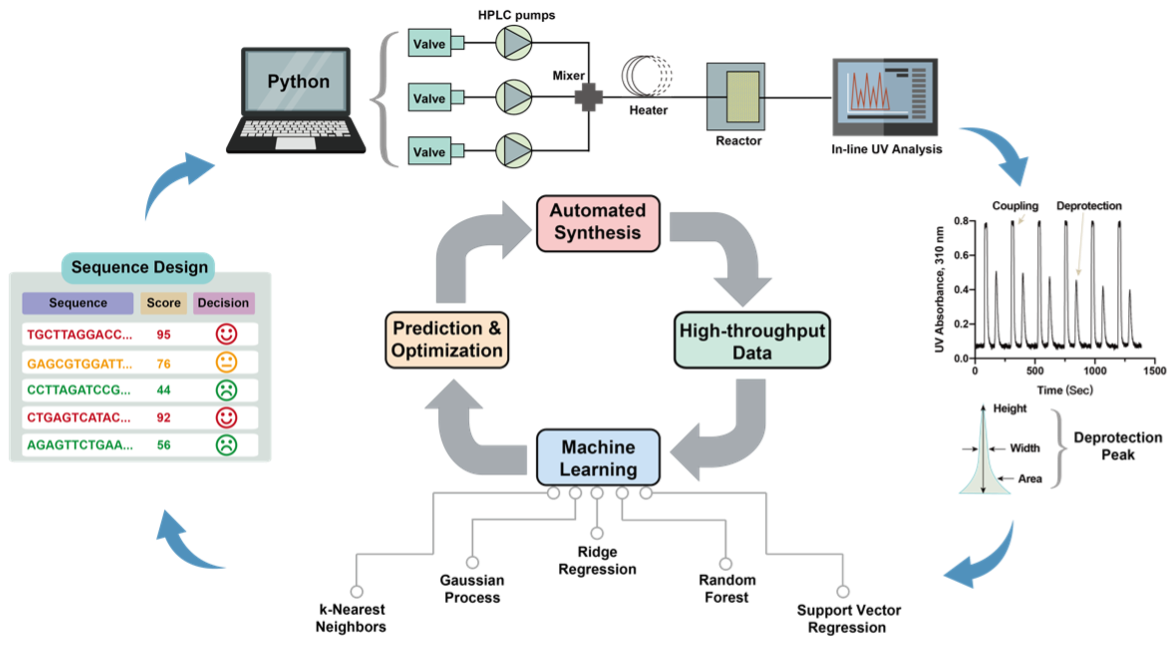
15. Li, C.;† Zhang G.;† Mohapatra S.; Callahan A.; Loas A.; Gomez-Bombarelli R.; Pentelute, B. L.* Machine Learning Guides Peptide Nucleic Acid Flow Synthesis and Sequence Design. Adv. Sci. (IF = 17.5), 2022, 9, 2201988.
Before ZJU
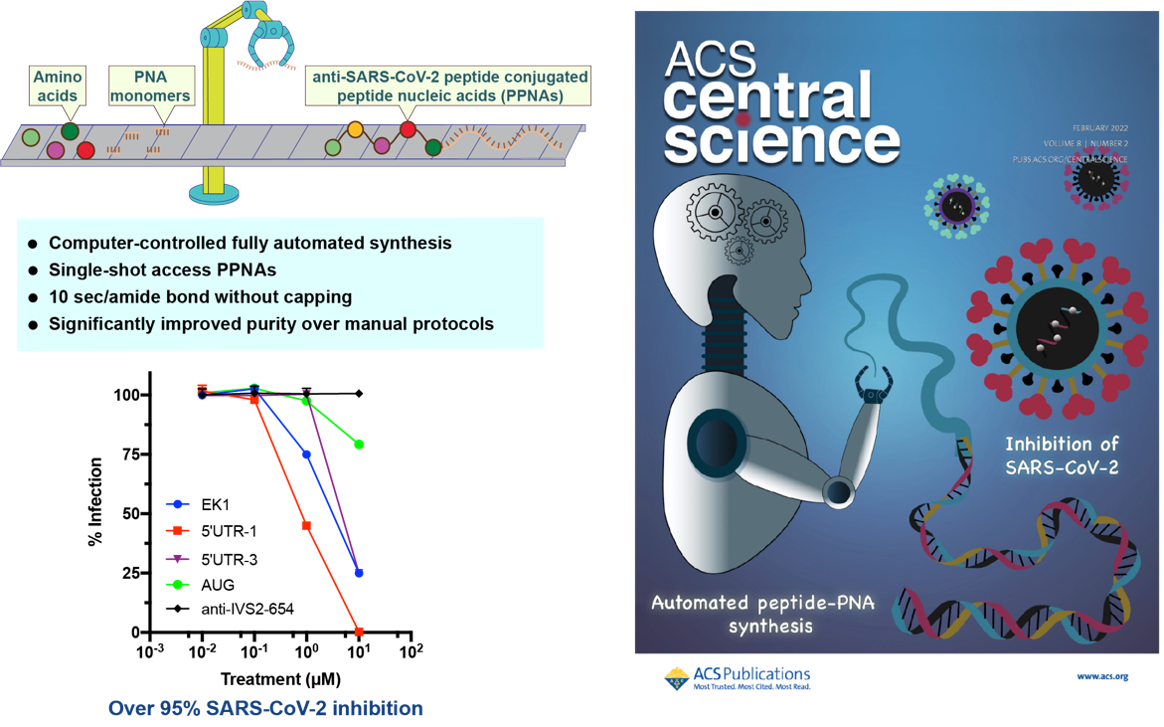
14. Li, C.; Callahan, A. J.; Phadke, K. S.; Bellaire, B.; Farquhar, C. E.; Zhang, G.; Schissel, C. K.; Mijalis, A. J.; Hartrampf, N.; Loas, A.; Verhoeven, D. E.; Pentelute, B. L.* Automated flow synthesis of peptide-PNA Conjugates. ACS Cent. Sci. (IF = 18.7). 2022, 8, 205-213.
*Highlighted by ACS Cent. Sci. as front cover art:
https://pubs.acs.org/toc/acscii/8/2
* Highlighted and reported by MIT news:
https://chemistry.mit.edu/chemistry-news/synthesis-too-slow-let-this-robot-do-it/
* Highlighted and reported by X-MOL:
https://mp.weixin.qq.com/s/M1MlFD3b-Hop7vHn8f91og
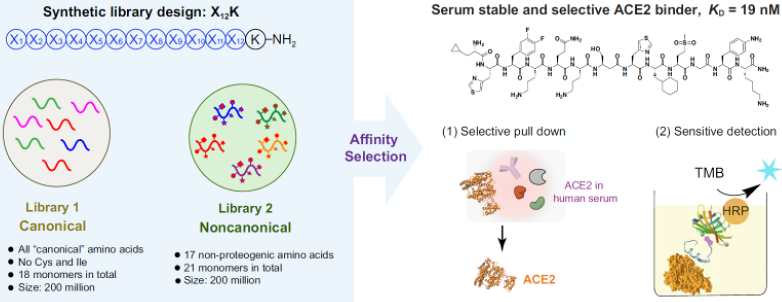
13. Zhang, G.;† Brown, J. S.;† Quartararo, A. J.; Li, C.; Tan, X.; Hanna, S.; Antilla, S.; Cowfer, A. E.; Loas, A. I.; Pentelute, B. L.* Rapid De Novo Discovery of High Affinity Noncanonical Binders for Human Angiotensin Converting Enzyme 2. Chem. Comm. (IF = 6.2). 2022. 1, 8.
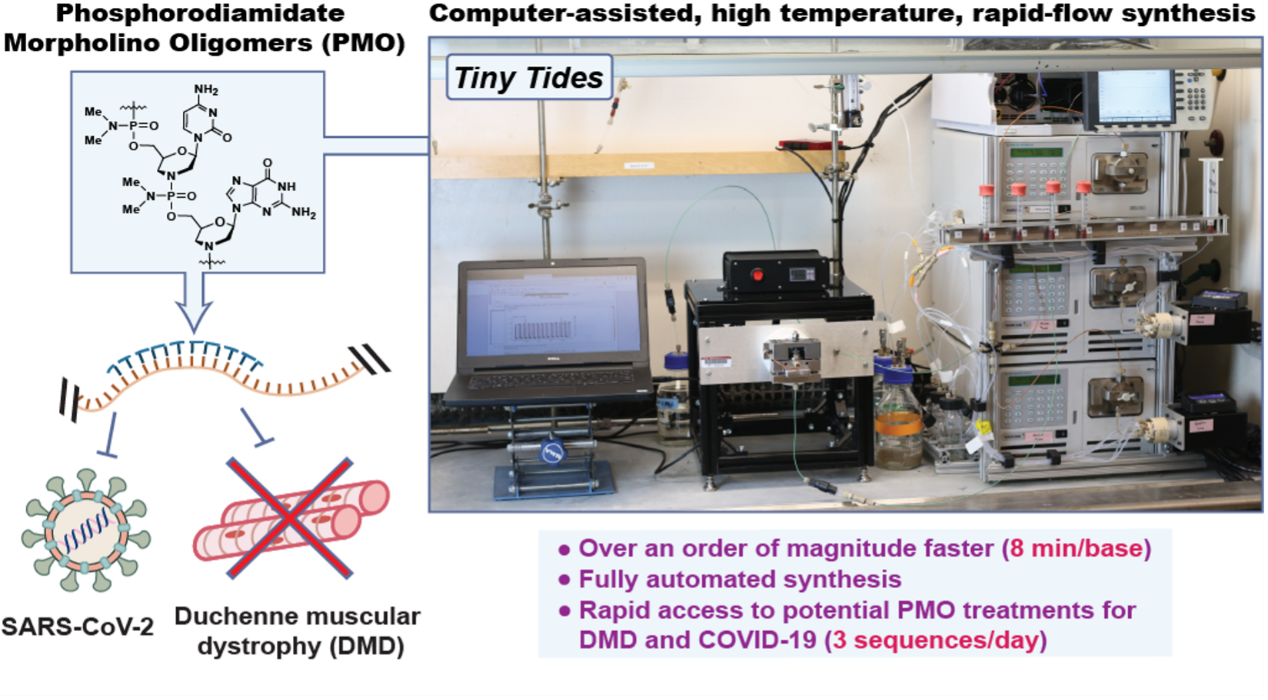
12. Li, C.;† Callahan, A. J.;† Simon, M. D.; Totaro, K. A.; Mijalis, A. J.; Phadke, K.-S.; Zhang, G.; Hartrampf, N.; Schissel, C. K.; Zhou, M.; Zong, H.; Hanson, G. J.; Loas, A.; Pohl, N. L. B.; Verhoeven, D. E.; Pentelute, B. L. Fully Automated Fast-flow Synthesis of Antisense Phosphorodiamidate Morpholino Oligomers. Nat. Commun. (IF = 17.7). 2021, 12, 4396.
11. Zhang, G.; Li, C.; Quartararo, A. J.; Loas, A. I.; Pentelute, B. L.* Automated Affinity Selection for Rapid Discovery of Peptide Binders. Chem. Sci. (IF = 9.9). 2021. 12, 10817.
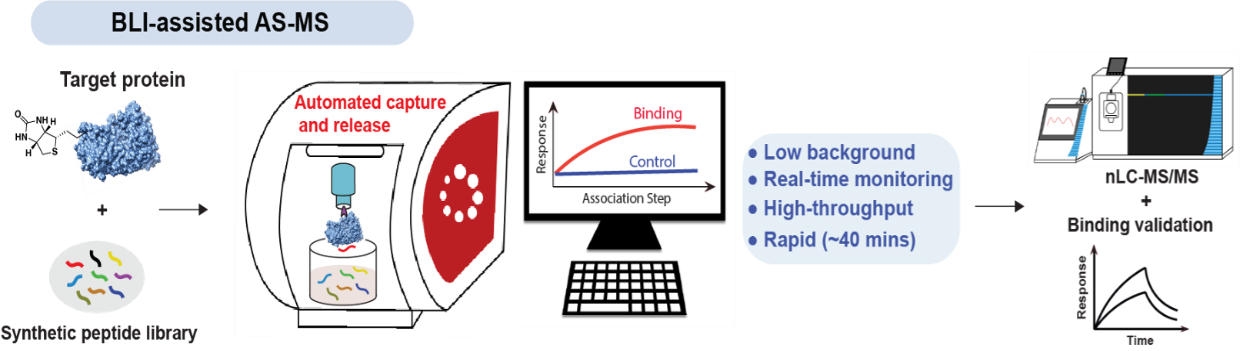
10. Li, C.;† Ragab, S. S.;† Liu, G.; Tang, W.* Enantioselective Formation of Quaternary Carbon Stereocenters in Natural Product Synthesis: A Recent Update. Nat. Prod. Rep. (IF = 15.1). 2020, 37, 276-292. (Invited Review).
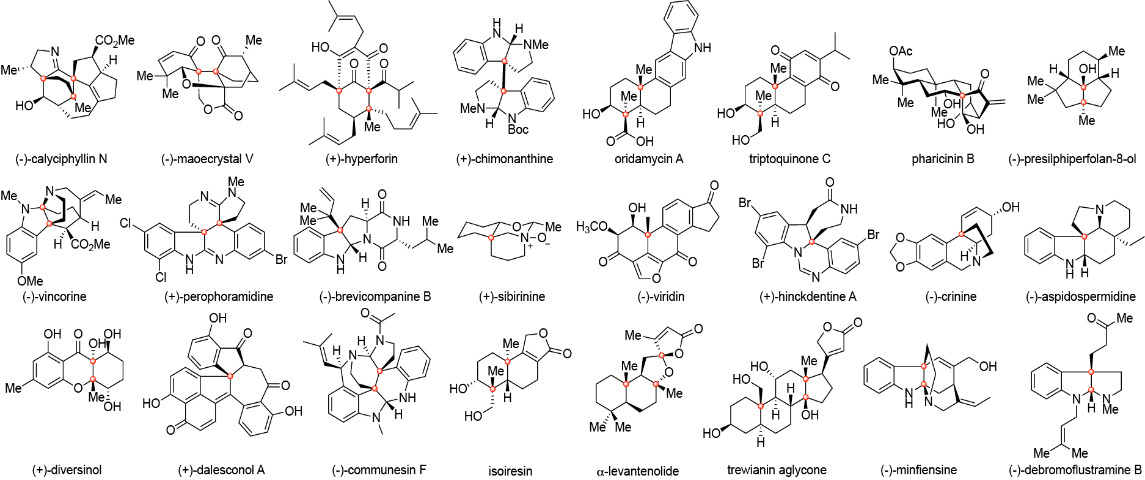
9. Li, C.; Liu, R. Y.; Jesikiewicz, L. T.; Yang, Y.; Liu, P.; Buchwald, S. L.* CuH-Catalyzed Enantioselective Ketone Allylation with 1,3-Dienes: Scope, Mechanism, and Applications. J. Am. Chem. Soc. (IF = 16.4). 2019, 141, 5062-5070.
*Highlighted by Synfacts
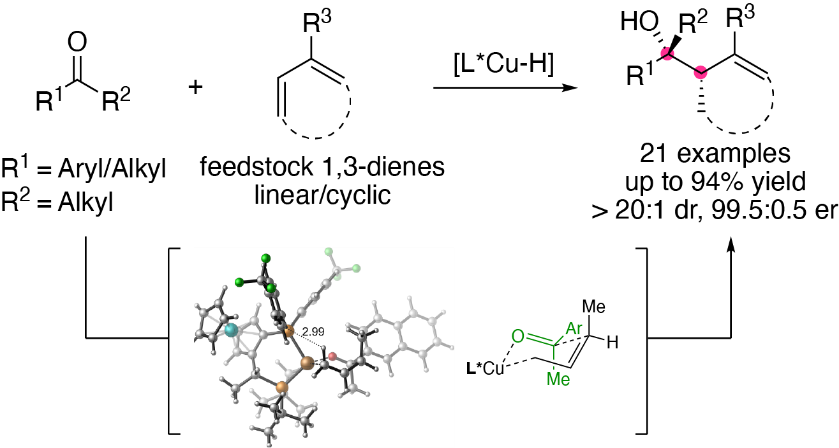
8. Li, C.;† Shin, K.;† Liu, R. Y.; Buchwald, S. L.* Engaging Aldehydes in CuH-Catalyzed Reductive Coupling Reactions: Stereoselective Allylation with Unactivated 1,3-Diene Pronucleophiles. Angew. Chem. Int. Ed. (IF = 16.8).2019, 58, 17074-17080.
*Highlighted by Synfacts
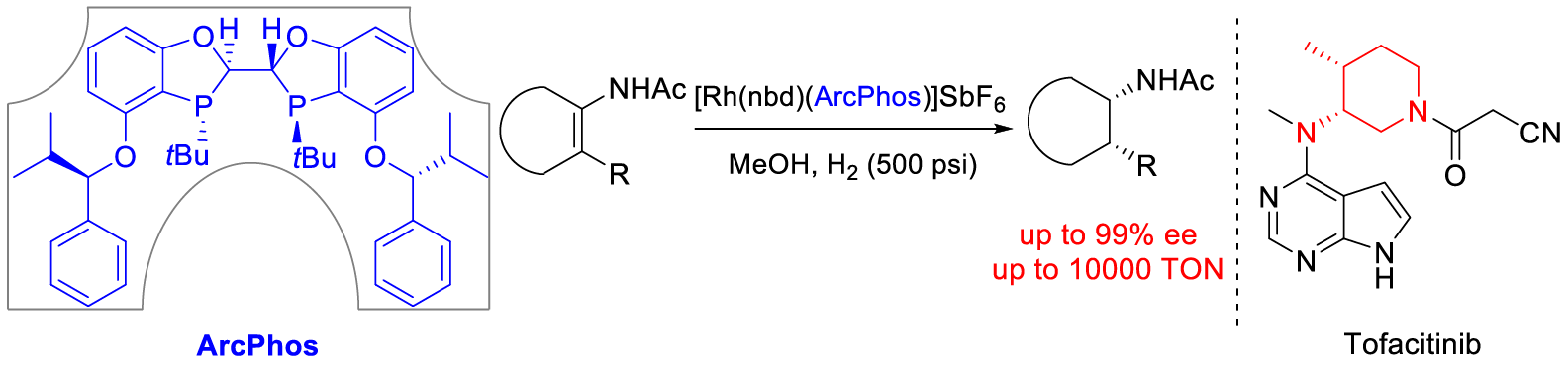
7. Li, C.;† Wan, F.;† Chen, Y.; Peng, H.; Tang, W.;* Yu, S.;* McWilliams, J. C.;* Mustakis, J.; Samp, L.; Maguire, R. J. Stereoelectronic Effects in Ligand Design: Enantioselective Rhodium-Catalyzed Hydrogenation of Aliphatic Cyclic Tetrasubstituted Enamides and Concise Synthesis of (R)-Tofacitinib. Angew. Chem. Int. Ed. (IF = 16.8). 2019, 58, 13573-13583.
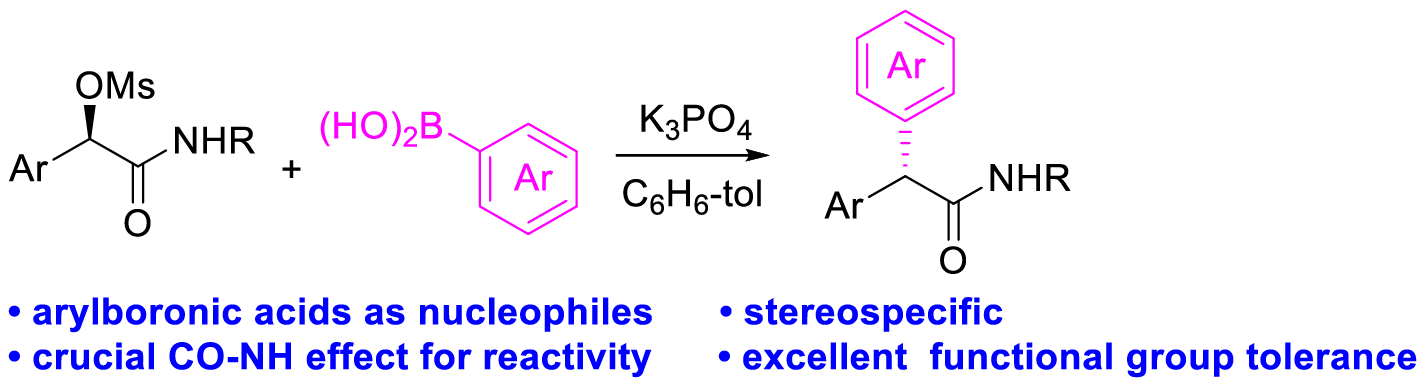
6. Tian, D.;†Li, C.;† Gu, G.; Peng, H.; Zhang, X.; Tang, W.* Stereospecific Nucleophilic Substitution with Arylboronic Acids as Nucleophiles in the Presence of a CONH Group. Angew. Chem. Int. Ed. (IF = 16.8). 2018, 57, 7176-7180.
*Highlighted by Synfacts
5. Li, C.; Zhang, Y.; Sun, Q.; Gu, T.; Peng, H.; Tang, W.* Transition-Metal-Free Stereospecific Cross-Coupling with Alkenylboronic Acids as Nucleophiles. J. Am. Chem. Soc. (IF = 16.4). 2016, 138. 10774-10777.
*Highlighted by Org. Process Res. Dev.

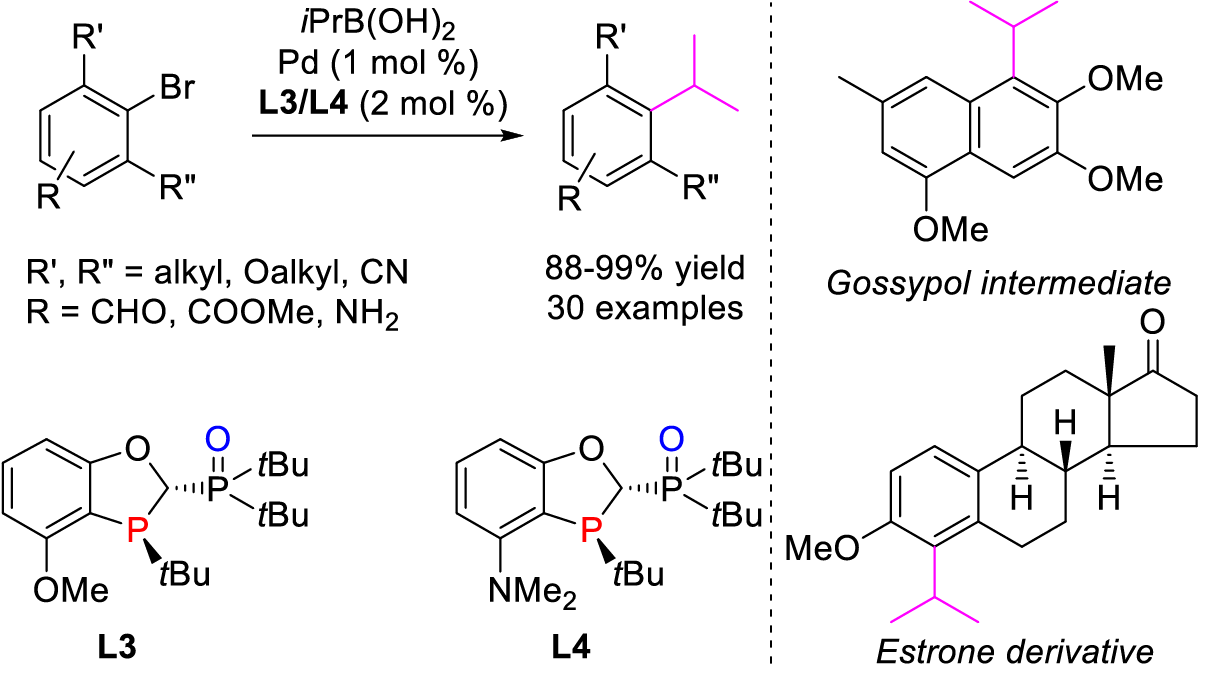
4. Li, C.; Chen, T.; Li, B.; Xiao, G.; Tang, W.* Efficient Synthesis of Sterically Hindered Arenes Bearing Acyclic Secondary Alkyl Groups by Suzuki-Miyaura Cross-couplings. Angew. Chem. Int. Ed. (IF = 16.8). 2015, 54, 3792-3796.
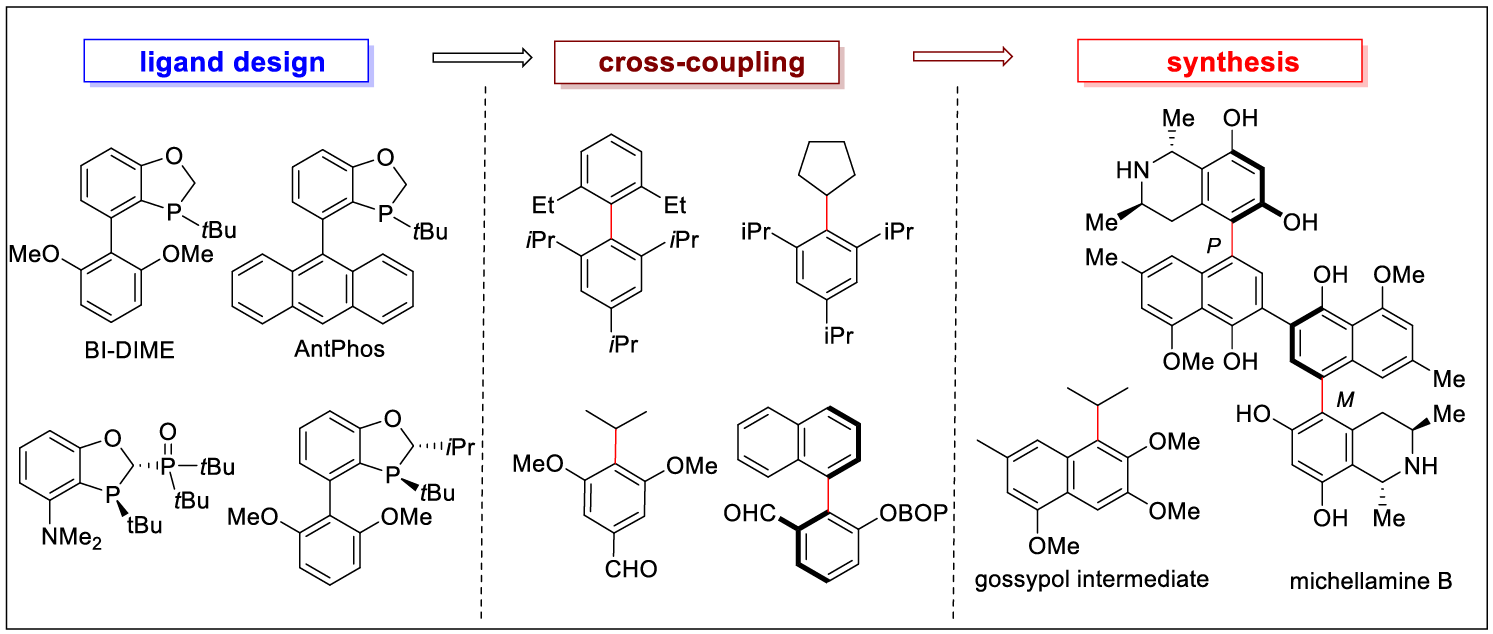
3. Li, C.; Chen, D.; Tang, W.* Addressing the Challenges in Suzuki–Miyaura Cross-Couplings by Ligand Design. Synlett., 2016, 27, 2183-2200. (Invited Review).
2. Li, C.; Xiao, G.; Zhao, Q.; Liu, H.; Wang, T.; Tang, W.* Sterically Demanding Aryl–alkyl Suzuki–Miyaura Coupling. Org. Chem. Front. (IF = 5.5). 2014, 1, 225.
*Highlighted as cover paper
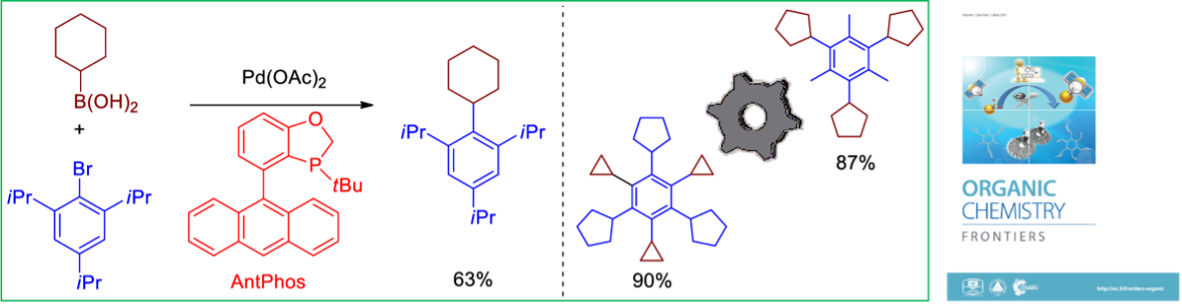
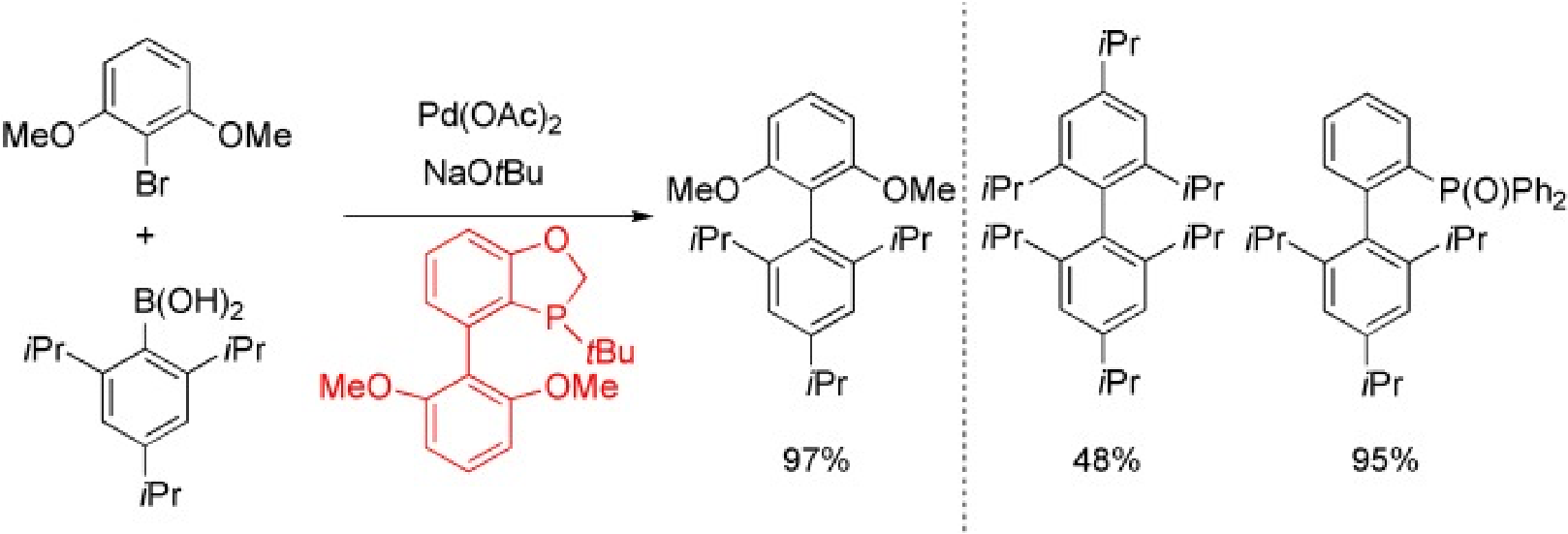
1. Zhao, Q.; Li, C.; Senanayake, C. H.; Tang, W.* An Efficient Method for Sterically Demanding Suzuki-Miyaura Coupling Reactions. Chem. Eur. J. (IF = 5.0).2013, 19, 2261-2265.
专利:
11. 李承喜、谢佩、于怡、万枫,一种芳基二胺固载连接子、其前体以及制备方法和应用,中国,ZL2024105731952, 2024.05.10.
10. 李承喜、潘有容、万枫,多功能可循环反应器,中国,ZL2023236291822, 2023.12.28.(授权)
9. 李承喜、万枫、潘有容,反应器,中国,ZL2023308626634,2023.12.28.(授权)
8. 李承喜、潘有容,适用于自动化流动合成的反应器,中国,ZL202223152425.3, 2023.04.17. (授权)
7. 李承喜、杨星星,一种染发剂初级中间体的合成方法,中国,202211503092.6, 2023.04.12. (授权)
6. 李承喜、左涛,自动化合成的可视化模块的构建方法及系统,中国,202211565728.X, 2023.04.12.
5. 李承喜、左涛,基于图学习的抗LRRK2 小分子药物预测和筛选方法,中国,202211571388.1, 2023.02.06.
4. 李承喜、宁左州,自动化高温流动合成天然环肽,中国,202211503753.5, 2022.11.29.
3. 李承喜、杨星星,一种染发剂初级中间体的合成方法,美国,PCT/CN2022/136573, 2023.04.20.
2. 汤文军、李承喜、万枫,金属络合物、制备方法和应用及其中间体,中国,ZL201710697385.5,2020.11.13. (授权)
1.汤文军、李承喜,手性膦配体以及包含膦配体的金属催化剂和它们的应用,中国,ZL201510063960.7,2015.02.06.
课题组主页链接:https://person.zju.edu.cn/chengxili
文章:
19. Zhu, C.; Zhang, C.; Shang, T.; Zhang, C.; Zhai, S.; Cao, L.; Xu, Z.; Su, Z.; Song, Y.; Su, A.; Li, C.; Duan, H.* GAPS: a geometric attention-based network for peptide binding site identification by the transfer learning approach. Brief. Bioinform., 2024, 25, bbae297.

18. Zhan, W.; Duan, H.; Li, C.* Recent Advances in Metal-Free Peptide Stapling Strategies. Chem Bio Eng., 2024, 1, 593–605. (Cover Art)


17. Miao, J.; Ghosh, A. P.; Ho, M. N.; Li, C.; Huang, X.; Pentelute, B. L.; Baleja, J. D.; Lin, Y.-S.* Assessing the performance of peptide force fields for modeling the solution structural ensembles of cyclic peptides. J. Phys. Chem. B2024, 128, 5281–5292.

16. Wu, J.; Yang, X.; Pan, Y.; Zuo,T.; Ning, Z.; Li, C.* Zhang, Z.* Recent Developments of Automated Flow Chemistry in Pharmaceutical Compounds Synthesis. J. Flow. Chem., 2023, 13, 385-404.

15. Li, C.;† Zhang G.;† Mohapatra S.; Callahan A.; Loas A.; Gomez-Bombarelli R.; Pentelute, B. L.* Machine Learning Guides Peptide Nucleic Acid Flow Synthesis and Sequence Design. Adv. Sci. (IF = 17.5), 2022, 9, 2201988.

Before ZJU
14. Li, C.; Callahan, A. J.; Phadke, K. S.; Bellaire, B.; Farquhar, C. E.; Zhang, G.; Schissel, C. K.; Mijalis, A. J.; Hartrampf, N.; Loas, A.; Verhoeven, D. E.; Pentelute, B. L.* Automated flow synthesis of peptide-PNA Conjugates. ACS Cent. Sci. (IF = 18.7). 2022, 8, 205-213.
*Highlighted by ACS Cent. Sci. as front cover art:
https://pubs.acs.org/toc/acscii/8/2
* Highlighted and reported by MIT news:
https://chemistry.mit.edu/chemistry-news/synthesis-too-slow-let-this-robot-do-it/
* Highlighted and reported by X-MOL:
https://mp.weixin.qq.com/s/M1MlFD3b-Hop7vHn8f91og

13. Zhang, G.;† Brown, J. S.;† Quartararo, A. J.; Li, C.; Tan, X.; Hanna, S.; Antilla, S.; Cowfer, A. E.; Loas, A. I.; Pentelute, B. L.* Rapid De Novo Discovery of High Affinity Noncanonical Binders for Human Angiotensin Converting Enzyme 2. Chem. Comm. (IF = 6.2). 2022. 1, 8.

12. Li, C.;† Callahan, A. J.;† Simon, M. D.; Totaro, K. A.; Mijalis, A. J.; Phadke, K.-S.; Zhang, G.; Hartrampf, N.; Schissel, C. K.; Zhou, M.; Zong, H.; Hanson, G. J.; Loas, A.; Pohl, N. L. B.; Verhoeven, D. E.; Pentelute, B. L. Fully Automated Fast-flow Synthesis of Antisense Phosphorodiamidate Morpholino Oligomers. Nat. Commun. (IF = 17.7). 2021, 12, 4396.

11. Zhang, G.; Li, C.; Quartararo, A. J.; Loas, A. I.; Pentelute, B. L.* Automated Affinity Selection for Rapid Discovery of Peptide Binders. Chem. Sci. (IF = 9.9). 2021. 12, 10817.

10. Li, C.;† Ragab, S. S.;† Liu, G.; Tang, W.* Enantioselective Formation of Quaternary Carbon Stereocenters in Natural Product Synthesis: A Recent Update. Nat. Prod. Rep. (IF = 15.1). 2020, 37, 276-292. (Invited Review).

9. Li, C.; Liu, R. Y.; Jesikiewicz, L. T.; Yang, Y.; Liu, P.; Buchwald, S. L.* CuH-Catalyzed Enantioselective Ketone Allylation with 1,3-Dienes: Scope, Mechanism, and Applications. J. Am. Chem. Soc. (IF = 16.4). 2019, 141, 5062-5070.
*Highlighted by Synfacts

8. Li, C.;† Shin, K.;† Liu, R. Y.; Buchwald, S. L.* Engaging Aldehydes in CuH-Catalyzed Reductive Coupling Reactions: Stereoselective Allylation with Unactivated 1,3-Diene Pronucleophiles. Angew. Chem. Int. Ed. (IF = 16.8).2019, 58, 17074-17080.
*Highlighted by Synfacts

7. Li, C.;† Wan, F.;† Chen, Y.; Peng, H.; Tang, W.;* Yu, S.;* McWilliams, J. C.;* Mustakis, J.; Samp, L.; Maguire, R. J. Stereoelectronic Effects in Ligand Design: Enantioselective Rhodium-Catalyzed Hydrogenation of Aliphatic Cyclic Tetrasubstituted Enamides and Concise Synthesis of (R)-Tofacitinib. Angew. Chem. Int. Ed. (IF = 16.8). 2019, 58, 13573-13583.

6. Tian, D.;†Li, C.;† Gu, G.; Peng, H.; Zhang, X.; Tang, W.* Stereospecific Nucleophilic Substitution with Arylboronic Acids as Nucleophiles in the Presence of a CONH Group. Angew. Chem. Int. Ed. (IF = 16.8). 2018, 57, 7176-7180.
*Highlighted by Synfacts

5. Li, C.; Zhang, Y.; Sun, Q.; Gu, T.; Peng, H.; Tang, W.* Transition-Metal-Free Stereospecific Cross-Coupling with Alkenylboronic Acids as Nucleophiles. J. Am. Chem. Soc. (IF = 16.4). 2016, 138. 10774-10777.
*Highlighted by Org. Process Res. Dev.

4. Li, C.; Chen, T.; Li, B.; Xiao, G.; Tang, W.* Efficient Synthesis of Sterically Hindered Arenes Bearing Acyclic Secondary Alkyl Groups by Suzuki-Miyaura Cross-couplings. Angew. Chem. Int. Ed. (IF = 16.8). 2015, 54, 3792-3796.

3. Li, C.; Chen, D.; Tang, W.* Addressing the Challenges in Suzuki–Miyaura Cross-Couplings by Ligand Design. Synlett., 2016, 27, 2183-2200. (Invited Review).

2. Li, C.; Xiao, G.; Zhao, Q.; Liu, H.; Wang, T.; Tang, W.* Sterically Demanding Aryl–alkyl Suzuki–Miyaura Coupling. Org. Chem. Front. (IF = 5.5). 2014, 1, 225.
*Highlighted as cover paper

1. Zhao, Q.; Li, C.; Senanayake, C. H.; Tang, W.* An Efficient Method for Sterically Demanding Suzuki-Miyaura Coupling Reactions. Chem. Eur. J. (IF = 5.0).2013, 19, 2261-2265.

专利:
11. 李承喜、谢佩、于怡、万枫,一种芳基二胺固载连接子、其前体以及制备方法和应用,中国,ZL2024105731952, 2024.05.10.
10. 李承喜、潘有容、万枫,多功能可循环反应器,中国,ZL2023236291822, 2023.12.28.(授权)
9. 李承喜、万枫、潘有容,反应器,中国,ZL2023308626634,2023.12.28.(授权)
8. 李承喜、潘有容,适用于自动化流动合成的反应器,中国,ZL202223152425.3, 2023.04.17. (授权)
7. 李承喜、杨星星,一种染发剂初级中间体的合成方法,中国,202211503092.6, 2023.04.12. (授权)
6. 李承喜、左涛,自动化合成的可视化模块的构建方法及系统,中国,202211565728.X, 2023.04.12.
5. 李承喜、左涛,基于图学习的抗LRRK2 小分子药物预测和筛选方法,中国,202211571388.1, 2023.02.06.
4. 李承喜、宁左州,自动化高温流动合成天然环肽,中国,202211503753.5, 2022.11.29.
3. 李承喜、杨星星,一种染发剂初级中间体的合成方法,美国,PCT/CN2022/136573, 2023.04.20.
2. 汤文军、李承喜、万枫,金属络合物、制备方法和应用及其中间体,中国,ZL201710697385.5,2020.11.13. (授权)
1.汤文军、李承喜,手性膦配体以及包含膦配体的金属催化剂和它们的应用,中国,ZL201510063960.7,2015.02.06.